To support the development of new therapies for autosomal dominant polycystic kidney disease, we offer a kidney-specific PKD1 knockout ADPKD mouse model. The age-dependent nature of cyst formation allows for tailoring of the model, enabling researchers to choose the most appropriate study design to fit their drug development pipeline.
Autosomal Dominant Polycystic Kidney Disease (ADPKD)
Autosomal dominant polycystic kidney disease (ADPKD) is the most common single-gene disorder and the most prevalent (4 to 10:10 000) progressive kidney disorder. The kidneys of ADPKD patients are affected by fluid-filled cyst formation and growth, ultimately resulting in kidney failure. However, the disease course of ADPKD patients tends to be highly variable with the age of onset ranging from early childhood to>80 years. Reports show that 50% of the patient population develops end-stage kidney failure by the time they reach 60 years of age (Bergmann et al., 2018).
Currently, the gold standard and only approved drug for ADPKD treatment is Tolvaptan. However, Tolvaptan induces serious side effects and does not allow long-term treatment. This limits the treatment of ADPKD to disease management strategies, highlighting the need for novel drugs targeting cystogenesis, that would slow down and/or halt further disease progression. Addressing this currently unmet medical need requires novel interventions to be tested in both a cost-effective and timely manner, which demands well-characterized, translational animal models of ADPKD.
ADPKD Pathophysiology and Relevant Targets
The main genes mutated in ADPKD patients are PKD1 or PKD2, with 85% of patients carrying the PKD1 gene mutation. A two-hit hypothesis has been proposed to explain the focal nature of cyst development, in which a germline mutation (first hit) in one of the two alleles together with a somatic mutation knocking out the second allele (second hit) lead to non-functional polycystin (PC) proteins. Although other genes have given mechanistic insights into the disease pathophysiology, the PKD1 gene is the most studied gene with respect to ADPKD. Moreover, the PKD1 protein interacts with the PKD2 protein, which is mutated in 15% of the ADPKD patient population (Bergmann et al., 2018). Therefore, models affecting the PKD1 gene represent an attractive tool for researchers involved in the search for novel therapeutics targeting ADPKD.
The gene product of PKD1 is PC1, which modulates several signaling pathways together with PC2, encoded by the PKD2 gene. Mutations in the PKD1 gene cause changes in PC1 expression, which interferes with several intracellular signaling pathways that are in control of cell proliferation, fluid secretion, and ciliary function, amongst others (Figure 1). The resulting dysregulation of cell proliferation and fluid secretion in the kidney leads to cyst development (1). The complex network of signaling pathways that are dysregulated in cystic kidneys provides many potential targets for therapeutic interventions (Figure 1).

FIGURE 1. Overview of autosomal dominant polycystic kidney disease (ADPKD) pathophysiology and main targets of potential treatments. Polycystin-1 and polycystin-2 (PC1 and PC2) are expressed in different subcellular locations (apical and basolateral membranes) where they are involved in the regulation of 1. cell proliferation 2. fluid secretion 3. ciliary function 4. cell-cell adhesion and 5. cell-matrix interactions (depicted as green boxes). Dysfunction in PC1 and PC2 leads to abnormal ciliary function and a decrease in intracellular calcium concentration which results in cAMP generation and mTOR activation, affecting cell proliferation and cyst development. The candidate drug targets (red boxes) include mTOR inhibitors (everolimus, rapamycin, metformin, curcumin), PC2 agonists, V2R antagonists (tolvaptan), somatostatin analogs, miRNA inhibitors, nrf2 activators, HDAC and CDKs inhibitors (roscovitine), EGFR inhibitors, CTFR modulators, and TNF-alpha inhibitors. Tolvaptan is a selective arginine V2R antagonist and decreases renal cAMP levels. TNF-alpha inhibitors target inflammation with a positive effect on pro-inflammatory markers found in ADPKD patients’ urine and renal cyst fluid. RNA-targeted therapies such as miRNA inhibitors, RNA-mediated interference, anti-sense oligonucleotides, and non-coding RNAs, can be used to regulate the expression of target mRNAs implicated in ADPKD and therefore their protein product. HDACs modulate gene expression by removing acetyl groups from histones and regulate a diverse array of intracellular pathways by acting on nonhistone proteins (e.g., cell cycle progression inhibition, downregulation of cAMP…). CFTR modulators act on CFTR, which is a chloride ion channel facilitating the transtubular chloride secretion to the cysts. Lastly, metabolic approaches are also an attractive way to target ADPKD, given that ADPKD cysts show dysregulated metabolism with a high rate of glycolysis and a low rate of mitochondrial oxidative phosphorylation.
Comprehensive Preclinical Testing of Novel ADPKD Drug Candidates using InnoSer’s gold standard ADPKD Mouse Model
Before novel therapeutics for ADPKD are approved for use in a clinical setting (i.e., by patients), rigorous preclinical testing in translationally relevant models is essential to demonstrate efficacy and safety, which are critical requirements for regulatory approval and successful IND dossier submission.
InnoSer’s inducible, kidney-specific Pkd1 knockout mouse model represents the gold-standard model for evaluating the pharmacokinetics, efficacy, and safety of various drug modalities, including small molecules, biologics, gene therapies, and metabolic interventions. Below, we highlight two cutting-edge therapeutic approaches gaining traction in the field of ADPKD, gene therapy and metabolic reprogramming, whilst outlining how InnoSer’s expertise using the ADPKD mouse model can serve to demonstrate preclinical efficacy driving preclinical-to-clinical translation.
Preclinical In Vivo Evaluation of Gene Therapies for ADPKD Using InnoSer’s Translational Mouse Model
Gene therapy is entering a new clinical era, with several approvals and more on the horizon bringing transformative potential to rare genetic diseases such as ADPKD. Given its monogenic nature and chronic progression, ADPKD is a strong candidate for gene-targeted therapeutic strategies. InnoSer’s nephrology team has ample experience in preclinical evaluation of gene therapies in the ADPKD mouse model across multiple delivery routes and disease stages.
While intravenous (i.v.) administration of viral vectors (typically AAV) is common, efficient kidney gene delivery is complicated by the kidney’s natural filtration barrier blocking vector passage, vector sequestration in glomeruli or recirculation reducing direct renal cell uptake, off-target transduction leading to high liver uptake and toxicity risk, immunogenicity from repeated dosing against vectors or transduced cells, and potential safety concerns such as insertional mutagenesis and acute toxicity.
Due to these factors, systemic gene delivery may have limited translational value for genetic renal diseases like ADPKD. Therefore, for drug developers using gene therapy as a therapeutic strategy for ADPKD treatment, InnoSer can advise on efficacy studies of gene therapy via local kidney injection using specialized surgical techniques (Figure 2). Alternative delivery strategies may also be considered, including ureteral administration that leverages the broader tubular epithelium for retrograde transduction, and the use of hydrodynamic pressure to facilitate vector penetration from the bloodstream into kidney tissue. InnoSer’s expert team can guide the selection and optimization of these techniques (including both IV and direct gene delivery) based on your gene therapy’s mechanism of action (MoA) and target cell type.

FIGURE 2. Surgical methods for targeted kidney gene therapy delivery. Surgical approaches to test novel gene therapy approaches include i) renal artery injection ii) venous injection iii) transparenchymal renal pelvis injection iv) hydrodynamic renal pelvis injection and v) intraparenchymal injection.
At InnoSer, our nephrology experts recognize that effective gene therapy for ADPKD requires a deep understanding of the kidney’s complex structure and the disease biology. Each delivery route transduces specific nephron compartments and renal cell types, which means that optimizing the gene delivery method to the therapeutic MoA and target cells is essential for success. Our expert team collaborates closely with you to optimize the dosing routes by considering critical factors such as:
- Transfection efficiency: Different delivery routes achieve variable transfection grades; selecting the optimal method is key to rescuing Pkd1 gene expression.
- Off-target effects: Minimizing liver transduction and systemic exposure reduces toxicity and immune responses.
- Vector size: Many gene therapy vectors exceed the pore size of glomerular slits, limiting passive entry into renal tissue.
- Injection-induced kidney damage: Some routes (e.g., clamping or injection-induced ischemia) may cause local tissue trauma, which can influence cyst development and disease progression in the ADPKD mouse model. Approaches like renal pelvis injection help reduce kidney trauma while improving vector retention.
- Target cell specificity: Considering which kidney cell types are being targeted guides the choice of delivery route and vector design.
- Pkd1 expression rescue titration: Both insufficient rescue and excessive overexpression of the Pkd1 gene can skew results or introduce additional pathology.
The age and developmental phase of the ADPKD mouse model may affect therapeutic timing and efficacy, informing whether gene therapy should be used prophylactically (i.e., P40 mouse model) or therapeutically (i.e., P18 and P40 mouse model; read more about the differences between the models in sections below). This flexibility to induce disease at different disease stages is critical for assessing timing-dependent gene therapy efficacy and optimizing delivery vectors, such as AAV or CRISPR/Cas9-based systems. Researchers can test the restoration of Pkd1 gene function in vivo, or delivery of therapeutic RNA molecules like antisense oligonucleotides (ASOs) and RNA interference strategies (i.e., siRNAs).
Delivery of therapeutic molecules packaged in delivery vehicles such as lipid nanoparticles (LNPs) can be tracked via bioluminescence imaging. Read more about InnoSer’s capabilities and view example studies for e.g., evaluating LNP biodistribution using bioluminescence imaging in this blog post.
Similarly, viral vectors like AAVs can be monitored using fluorescent reporter tags such as GFP, enabling visualization of transduction efficiency and tissue targeting.
By closely taking into account your novel ADPKD therapy’s MoA, we collaborate closely with you to design and optimize delivery methods that maximize transduction efficiency while minimizing adverse effects, ensuring the best possible translational outcomes for your ADPKD gene therapy programs.
Targeting Metabolic Reprogramming in ADPKD: A New Therapeutic Avenue
Recent advances in the understanding of ADPKD pathophysiology have revealed that cystic epithelial cells and tissues undergo a metabolic reprogramming reminiscent of the Warburg effect, shifting from oxidative phosphorylation to aerobic glycolysis (Kanhai et al., 2023). This altered metabolic state presents a novel opportunity for both therapeutic and dietary metabolic targeting in ADPKD.
At InnoSer, our ADPKD mouse model serves as an ideal platform to investigate compounds that aim to reverse or exploit these metabolic abnormalities. Metabolic reprogramming interventions, including mTOR inhibitors (e.g., rapamycin, everolimus), AMPK activators (e.g., salsalate), glycolysis inhibitors (e.g., 2-DG), and mitochondrial function modulators, can be tested across different disease progression models (P10, P18, P40) to determine the optimal therapeutic approach.
To illustrate the potential of metabolic activators in ADPKD, here we present a focused case study featuring our in-house validation studies on salsalate, an AMPK activator that has demonstrated promising results, consistent with the current state-of-the-art literature on salsalate, metabolic reprogramming and drug repurposing for ADPKD.
Case Study: Salsalate as a Metabolic Activator in ADPKD
Salsalate, a pro-drug dimer of salicylate belonging to the family of NSAIDs, activates AMPK through direct interactions with the drug-binding domain of the AMPK beta-1 isoform. As such, Salsalate arises as a key candidate for drug repurposing in ADPKD.
ADPKD mice (P18 mouse model; Pkd1 KO was induced from PND18) treated with Salsalate (0.25% in food for 10 weeks) show a significant decrease in kidney volume (assessed via ultrasound; data shown corrected for body weight; Figure 3), blood urea levels (Figure 4) as well as reduced cystic kidney disease severity (cystic index) compared to vehicle control mice (P=0.0001; Figure 5). In line with our results, previous research has shown that Salsalate has slowed PKD progression in ADPKD mouse models by improving mitochondrial function and reducing inflammation (Leonhard et al. 2019; Song et al. 2023; Kanhai et al. 2023).

FIGURE 3. Salsalate treatment (0.25% in food) reduces kidney volume (corrected for body weight) in the ADPKD mouse model. Compared to Pkd1 KO mice administered with vehicle (food pellets), Salsalate–fed Pkd1 KO mice have a significantly lower kidney volume at post-natal day (PND) 97 (P<0.001).

FIGURE 4. Salsalate treatment reduces the blood urea levels in the ADPKD mouse model. Compared to Pkd1 KO mice administered with vehicle (food pellets), Salsalate-administered Pkd1 KO mice show a progressive lower blood urea levels (mmol/l), indicative of a stable kidney function (PND74: *P=0.0155; PND98: **P=0.0028; PND102: **P=0.0010; PND109: **P=0.0014, vehicle-treated vs Salsalate treated Pkd1 KO mice; mixed effect analysis).
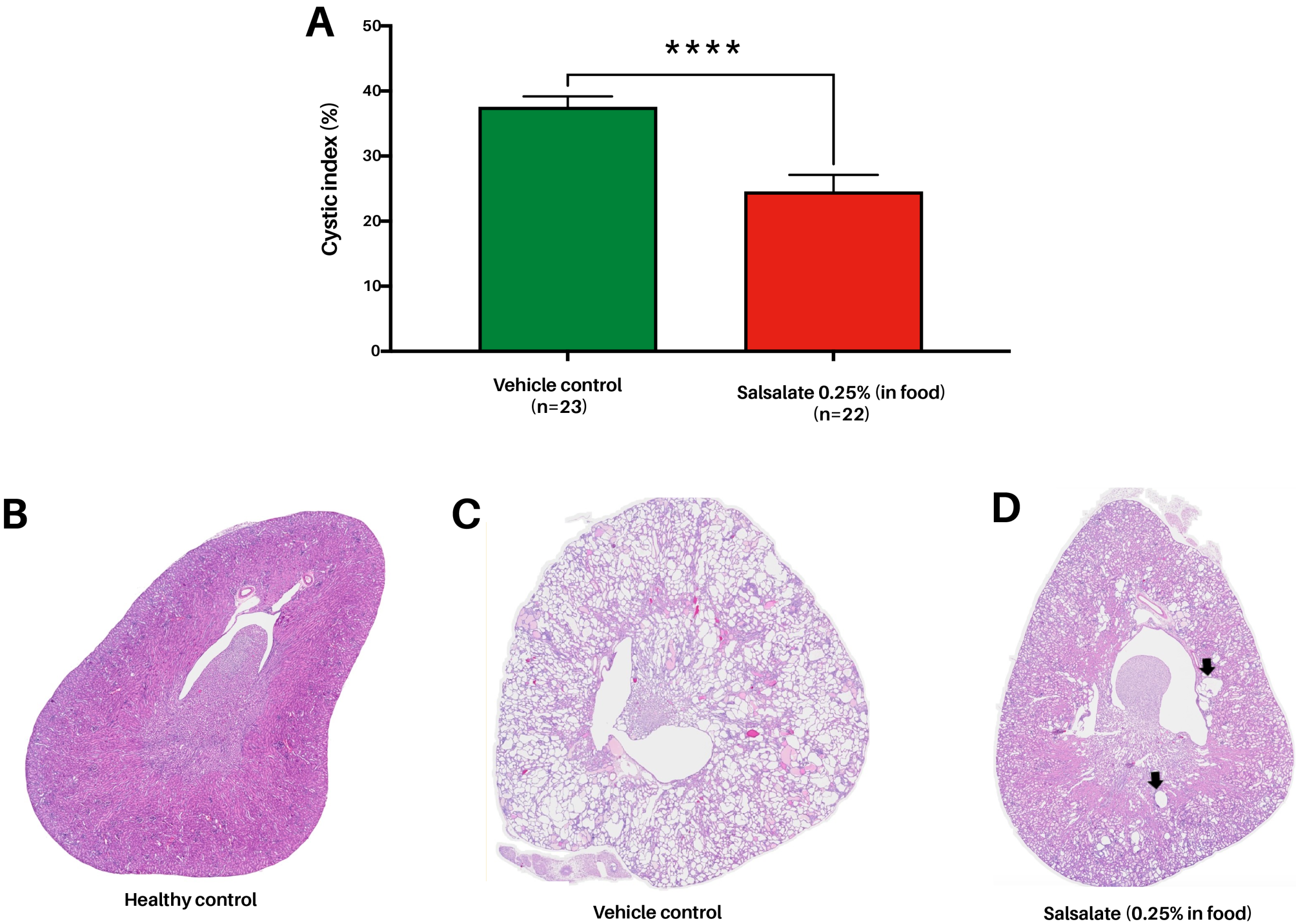
FIGURE 5. Salsalate treatment (0.25% in food) reduces cystic index (%) in the ADPKD mouse model. (A) Compared to Pkd1 KO mice administered with vehicle (food pellets), Salsalate-administered Pkd1 KO mice show a significant decrease in cystic index (P=0.0001), indicative of improvement of the pathophysiological kidney disease. (B) Representative histological image of kidney obtained from healthy mouse. (C) Representative histological image of kidney obtained from Pkd1 KO vehicle-treated mouse. (D) Representative histological image of kidney obtained from Pkd1 KO Salsalate-treated animal. In comparison to vehicle-treated animals, Salsalate-treated mouse have smaller cysts with lower distribution (arrows).
As research expands beyond traditional small molecules, InnoSer’s ADPKD research platform offers you with a flexible, validated platform for testing a wide array of therapeutic strategies, from gene-targeted interventions to metabolic reprogramming. Whether you’re working on RNA-based drugs, gene correction technologies, or energy metabolism modulators, our team supports tailored study designs to accelerate your path from discovery to clinical translation. Contact our team to discuss your therapeutic pipeline and explore how we can help generate robust, translatable in vivo data using our ADPKD model.
Validated, translationally relevant and customizable ADPKD mouse model to study novel ADPKD treatments
At InnoSer, we offer a uniquely engineered inducible, kidney-specific Cre(lox,lox)–Pkd1 knock-out (KO) ADPKD mouse model (Lantinga van Leeuwen et al., 2007). Conditionally disrupting Pkd1 gene expression prevents embryonic lethality of mice that occurs with Pkd1 germline deletion due to a severe cystic phenotype. The inducible feature of this model allows a disruption of PKD1 gene expression at specifically chosen time points, both in developing and adult mice. This not only allows a well-controlled analysis of ADPKD pathogenesis, but also the creation of different ADPKD progression models, thanks to the characteristic age-dependent cyst formation observed in our model.
When conducting preclinical research using our model, there are three time points at which the KO can be induced, referring to the post-natal day (P) at which tamoxifen-induced Pkd1 gene disruption is performed; P10, P18 and P40. As the kidney tissue is found in different proliferative stages in the juvenile and adult mice, differences in susceptibility to cystogenesis are observed in each approach. Consequently, each timing of the Pkd1 gene disruption has a major effect on the severity and progression of the cyst development (Figure 6). Therefore, each study time point presents a unique model with its own benefits and considerations, which we explain below.

FIGURE 6. H&E-stained kidney sections depicting the differences in renal cystic phenotypes between P10 and P18 ADPKD mouse models. (A-C) Pkd1 knock-out (KO) at post-natal day 10 (P10) shows fast progression to cystogenesis in the outer medulla compared to the other kidney regions. Additionally, this model shows rapid formation of multiple large cysts within a short period. Treatment with previously validated drug against ADPKD, such as Everolimus shows a significant reduction in the cystic phenotype. (D-F) In contrast to the P10 model, the P18 model shows synchronized formation of multiple, smaller cysts throughout the whole kidney region. Treatment with previously validated drug against ADPKD, such as Tolvaptan shows a significant reduction in the cystic phenotype.
P10 study: Perform Fast Lead Compound Screening Studies using the ADPKD Mouse Model
P10 studies offer researchers a compound screening platform, highly suitable for quick and robust testing, ideal for pharmacokinetic as well as efficacy and tolerability studies with multiple lead compounds. Moreover, it has been shown that the early onset of ADPKD in neonatal and juvenile patients leads to relatively large cysts, explained by the rapid cyst growth in the developing kidneys, which is also observed in the P10 model.
Disruption of Pkd1 gene expression at P10 in the ADPKD mouse model leads to the rapid and massive development of cysts in the distal segment of the nephron (Figure 6), since the renal epithelium is still in a proliferative stage (Figure 7). Thanks to its short study nature (in-life phase lasts 4 weeks), P10 studies are the most cost- and time-efficient to perform, in comparison to P18 and/or P40 studies.

FIGURE 7. Differences in renal proliferative state of the different ADPKD disease progression models and the gold-standard treatment response in the ADPKD mouse model. Histological images show areas of epithelial cell nuclei, positive for the proliferation marker Ki67. (A) The renal tubular epithelium of the P10 model shows significant proliferation around the cysts, indicated by the strong Ki67 expression (shown by the zoomed-in section). (B) Treatment with everolimus reduces the number of proliferative cells around the cysts, indicated by the weak Ki67 staining. (C) In contrast to the P10 model, the P18 model shows a lower number of cells undergoing proliferation around the cysts. Differences in cyst development and growth between the P10 and P18 models are thus likely related to the differences in proliferation. (D) Following treatment with tolvaptan, kidneys from animals of the P18 model showed decreased cell proliferation, indicated by a weak and/or absent Ki67 expression. Scale bars are shown for each respective image.
P18 & P40 studies: Perform Long-term Studies using the ADPKD Mouse Model
P18 and P40 studies are typically preferred by researchers who wish to evaluate the efficacy of their compound in a more clinically relevant set-up. It has been reported that individuals with adult-onset ADPKD show cyst formation and growth at a much slower rate compared to juvenile-onset ADPKD patients (Leonhard et al., 2016). Knocking out Pkd1 at P18 (in-life phase lasts approx. 18 weeks) and P40 (in-life phase lasts around 27 weeks) leads to a relatively slow cyst development and progression, affecting all segments of the nephron (Figure 6). This resembles the course of the pathophysiology in adult disease onset ADPKD. P18 and P40 studies can additionally provide a platform for researchers looking to perform chronic treatment studies, providing the opportunity to also detect any possible long-term side effects.
Readout Selection: How to Measure Disease Progression in the ADPKD mouse model?
At InnoSer, we use several readouts to assess the disease progression and the effects of novel targeted ADPKD compounds using the ADPKD mouse model. General pharmacokinetic studies allow the assessment of biodistribution of the compounds of interest, given that kidney-specific compound administration is crucial to prevent systemic toxicity associated with long-term therapy in ADPKD patients. We have highlighted the importance of performing PK/PD and tolerability studies in the ADPKD model in this article. Kidney volume is an important predictor of ADPKD progression in the patient population and is therefore also highly translational in the preclinical testing of novel therapeutics. Leveraging our in vivo capabilities, we perform ultrasound kidney volume measurements to gain translationally relevant longitudinal data. More importantly, the total kidney volume (TKV) is considered a surrogate biomarker for a decline in kidney function in ADPKD patients, approved by EMA and the FDA. The increase in kidney volume is related to a decline in the glomerular filtration rate (GFR), which provides highly relevant information about the progression of the disease. At InnoSer, GFR is assessed transdermally, allowing the collection of longitudinal assessment of renal function (Figure 8). On the other hand, blood creatinine and urea measurements provide more general kidney function parameters and are less expensive to perform than GFR.
In addition to kidney volume and function, cystogenesis, and other associated pathological lesions, as well as the effect of novel treatments on inhibiting or halting the development and/or further growth of cysts can be assessed by routine H&E stains. The analysis of the total area of cysts within the total area of the kidney, referred to as a cystic index (to read more about the application of cystic index in the ADPKD model you can view our previous article by clicking here), allows us to quantify the process of cystogenesis following routine H&E staining. Finally, specific stainings can be used to assess fibrosis (PSR, Trichrome, alpha-SMA), proliferation (Ki67), and cyst growth (Cyclin D1) to gain additional insight into the therapeutic mechanism of investigational drugs.

FIGURE 8. Transdermal GFR assessment shows decline in kidney function in ADPKD mice. Over time, we observe a significant decrease in the GFR of the PKD group in comparison to the healthy control group (post-natal day [PND] 81 PKD group vs PND115 PKD group *P=0.0096; PND102 PKD group vs PND115 PKD group **P=0.0172; PND115 healthy vs PND115 PKD group ***P=0.0016; M±SEM; dots represent individual animals), confirming the loss of renal function in PKD mice and the suitability of this method in efficacy studies.
Choosing the Right Study Type for Your Research with the ADPKD Mouse Model
The most appropriate study type and design ultimately depend on the specific aims of the research question. Each model has its characteristics, such as the age and rate of disease progression, the affected nephron segment, the number of affected nephrons, and the rate of cyst formation and growth. Factors such as the pharmacological properties of the compound being tested, as well as target protein expression in different nephron segments and cystic epithelia, need to be considered. To learn more about ensuring that you work with validated kidney disease models, you can read our previous article which highlights main questions that you should determine prior to commencing studies using kidney disease models.
Consulting with our nephrology study experts will allow you to carry out tailored studies while collecting the most study-appropriate data. We also advise you on the most optimal model selection, taking in your budget and study timelines.