Transactivating response region (TAR) DNA binding protein (TDP-43) is a nuclear protein involved in regulating RNA transcription, splicing, trafficking, and microRNA biogenesis. TDP-43 is a major component of neuronal and glial inclusions in both the brain and the spinal cord, found in approximately 95-97% of ALS cases and in ~50% of FTD cases, making TDP-43 a major therapeutic target across the ALS-FTD disease spectrum.
Currently, a multitude of TDP-43 mouse models have been developed; broadly speaking, transgenic TDP-43 mouse models can be grouped into high TDP-43 overexpression and low/endogenous TDP-43 overexpression mouse models (for a comprehensive overview of all TARDBP mouse models, see Stephenson et al., 2017).
TDP-43(Q331K) mouse model used for preclinical efficacy studies at InnoSer
InnoSer currently works with the TDP-43Q331K mouse model. The TDP-43(Q331K) mouse model carries a human ALS-associated Q331K mutation (Sreedharan et al., 2008) in the C-terminal domain of human TDP-43 directed to the brain and spinal cord by the mouse prion protein promoter (PrP), a region critical for protein regulation and RNA binding.
This single transgenic TDP-43 mouse model of ALS and FTD has been shown in literature (Arnold et al., 2013; Watkins et al., 2021) and confirmed by InnoSer’s validation experiments (link to figures here and here) to recapitulate the pathophysiological disease features (motor unit dysfunction, neuronal damage marked by plasma NfL, and robust motor function deficits), of human TDP-43 proteinopathy. Although this model shows cytoplasmic TDP-43 accumulation (Figure 1), robust motor neuron disease features develop starting at 4 months of age without robust cytoplasmic TDP-43 aggregation and nuclear clearing (Arnold et al., 2013; Wakins et al., 2012; Mitchell et al., 2015).
Utilizing the TDP-43(Q331K) mouse model to perform longitudinal studies and evaluate rescue of motor function deficits across the ALS/FTD disease continuum
This single transgenic TDP-43 mouse model avoids artefacts (such as premature lethality) associated with other TDP-43 mouse models featuring high TDP-43 overexpression leading to insoluble cytoplasmic TDP-43 inclusions and TDP-43 nuclear clearing (such as observed in the TDP-43 TAR4/4 mouse model; Wils et al., 2010) or the double transgenic TDP-43 mouse model described by Mitchell et al., 2015), allowing you to perform longitudinal studies (starting from 2-4 months of age when mice display early stage disease phenotype, or from 4-6 months of age when mice display robust motor function deficits).
Importantly, cytoplasmic aggregation of TDP-43 into insoluble inclusions in cortical and spinal neurons has been observed in this mouse model at 24 months of age (see supplementary figure 4 in Mitchel et al., 2015), making it more suitable for longitudinal studies examining therapeutic rescue of motor function deficits in the setting of ALS and/or FTD.
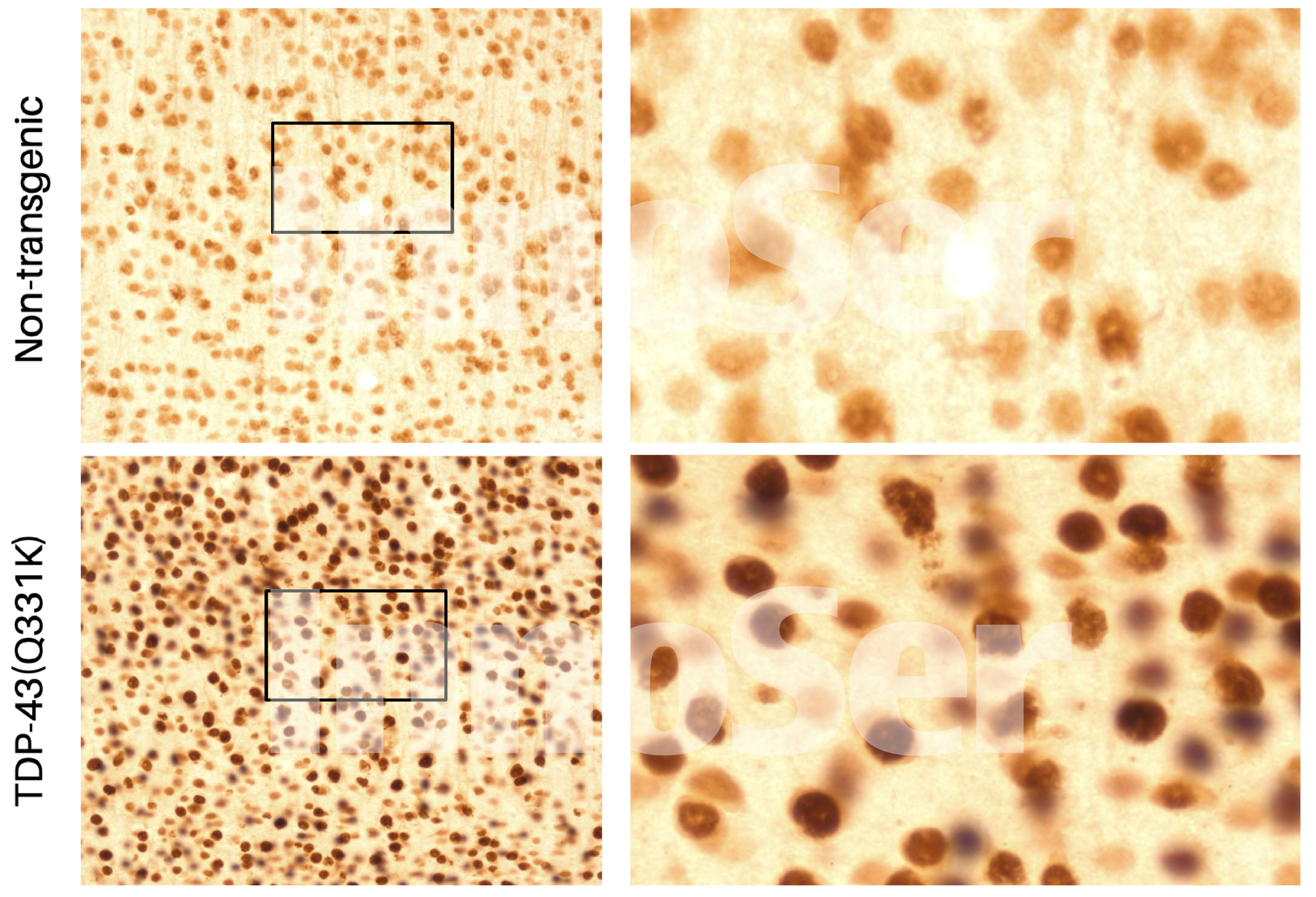
FIGURE 1. The single transgenic TDP-43(Q331K) mouse model develops robust motor neuron disease pathophysiology features (see InnoSer’s validation data here and here) in the absence of overt cytoplasmic mislocalization and cytoplasmic TDP-43 aggregates/ inclusions and TDP-43 nuclear clearing, in line with literature (Arnold et al., 2013; Watkins et al., 2015; Mitchel et al., 2015) and other single transgenic TDP-43 mouse models such as the TAR6/6 TDP-43 mouse model (Wils et al., 2010). InnoSer’s in-house validation of the TDP-43 mouse model included a qualitative assessment (performed in the same staining session and imaged under the same conditions) of TDP-43 (mouse and human TDP-43) cellular localisation, confirming an increase in the level of both cytoplasmic and nuclear TDP-43 compared to non-transgenic littermates. Note: Non-transgenic TDP-43 littermates show a faint nuclear TDP-43 signal due to the presence of endogenous mouse TDP-43.
Responsiveness to drug treatment of the TDP-43(Q331K) mouse model
While the TDP‑43Q331K transgenic mouse model has been extensively characterized for progressive motor and neuromuscular deficits, published studies to date have not reported standard therapeutic efficacy experiments in this specific model. Unlike SOD1 models (where drugs like riluzole, edaravone, or Tofersen for SOD1 ALS have been used as positive controls in some studies), there is currently no universally accepted comparator molecule and/or standard-of-care therapeutic that has shown robust disease modification in the TDP‑43(Q331K) mouse model.
However, several recently published studies have demonstrated that the TDP-43(Q331K) mouse model is pharmacologically responsive to experimental interventions. For example, a pre-print has reported that treatment with dasatinib and quercetin, a senolytic combination, improved motor performance and reduced disease-associated phenotypes in TDP-43Q331K mice (Viteri et al., 2025). In separate studies, M102, a CNS-penetrant small-molecule electrophile that activates the NRF2-ARE and heat-shock response pathways, has also been shown to ameliorate disease-related functional deficits in this mouse model of ALS and FTD (Keerie et al., 2025).
Model selection for aggregation-targeting therapeutic strategies
The TDP-43Q331K mouse model is well-suited for evaluating therapeutic strategies targeting early and progressive disease mechanisms, including preservation of motor function, neuronal resilience, RNA dysregulation, proteostasis, and cellular stress pathways.
For programs targeting TDP-43 mislocalization and cytoplasmic aggregation, nuclear clearing, and alternative TDP-43 mouse models that develop robust cytoplasmic inclusions and nuclear depletion may be more appropriate. Such aggregation-focused phenotypes have been described in the literature for e.g., in the double-transgenic TDP-43WTxQ331K model where TDP-43 proteinopathy pathology is present (see figure 5 of Mitchell et al., 2015). As reported in this study, the addition of a human TDP-43, although wild-type, triggered massive TDP-43 aggregation in the TDP-43Q331K mice and increased toxicity (mice die around the age of 8 weeks). Authors have further hypothesized that Q331K aggregates provide a potent seed for the recruitment of human WT and endogenous mouse TDP-43 that results in nuclear clearing and rapid neurodegeneration.
Reach out to our expert scientific team to learn more about aggregation-focused TDP-43 mouse models. Our team will work together with you to identify the most suitable model, taking into account your needs and your therapeutics’ MoA.
Be the first to know – get this news straight to your inbox
InnoSer provides a variety of validated in vitro and in vivo screening tests for psychiatry and neurology. If you require additional information, feel free to reach out, and we will respond within a few days.
We provide a monthly update from our neurology labs containing scientific insights into our research services. To get news straight to your inbox please sign up here.